Celitement releases less carbon dioxide during its manufacture than classic Portland cement klinkers.
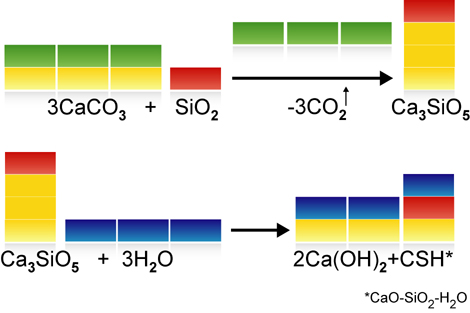
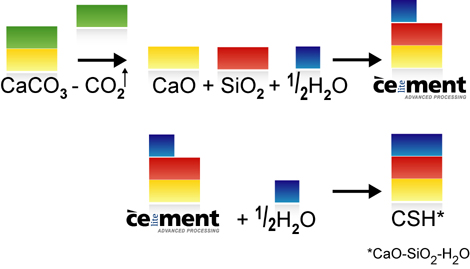
Around 770-870 kg of carbon dioxide is generated by the manufacture of a tonne of ordinary Portland cement clinker. Around 75% of this is caused by the deacidification (or calcination) of the main raw material, calcium carbonate (CaCO3). Less limestone is needed for the manufacture of Celitement (see video clip on material balance), so less CO2 is released. In addition to cement clinkers, though, modern cements also contain additives such as gypsum, limestone meal, granulated slag, fly ash or natural pozzolans. These additives can also be combined with Celitement and enable the CO2 intensity to be even further reduced. When comparing the GWP (Greenhouse Warming Potential) of new binding agents with traditional cements, the basis for comparison selected must be taken into account. Is the comparison based on pure Portland cement clinkers, Portland cement (CEM I), a composite cement (CEM II) or even what is known as CEM VI cement? Our benchmark is Portland Cement Clinker.